SCTB

The Service for Clinical Trials and Biometrics (SCTB) integrates training activity, methodological development, research activity and teaching, with the activities typically carried out by the promoters of clinical research projects or by organizations that manage clinical trials in the name and on behalf of the promoters. It deals with the coordination and management of projects in their entirety or specific activities or functions, from clinical trials, to clinical investigations with pre-market and post-market medical devices, to interventional studies and observational studies in wide specialized clinical fields, including those related to the field of application of nursing, epidemiology and health in general and studies on supplements, nutrients, foods and foods for special purposes.
SCTB deals with fund rising, grant application, study design, thus ensuring a scientific and management coverage that also includes administrative, contractual, fund management, data and results reporting aspects, from the conception of the research project to the publication of the results. SCTB supports the design of clinical and non-clinical research projects, revises or drafts research protocols according to international standards, designs and builds data collection systems (case report forms – CRF – also electronic – electronic data capture – EDC) adapted to the applicable national and international legislation, but also deals with GCP training, regulatory management of clinical trials, management of pharmacovigilance activity, also in the field of EudraVigilance.
SCTB advises on the different aspects related to clinical research in the fields of data management and protection of privacy, risk management and pharmacovigilance, as well as in aspects related to the application of ethics to clinical research.
SCTB also deals with the production of standalone surveys or related to research projects, systematic literature reviews and standalone or instrumental metanalyses to the definition of clinical and non-clinical research projects as well as in support of the production of guidelines and consensus conferences.
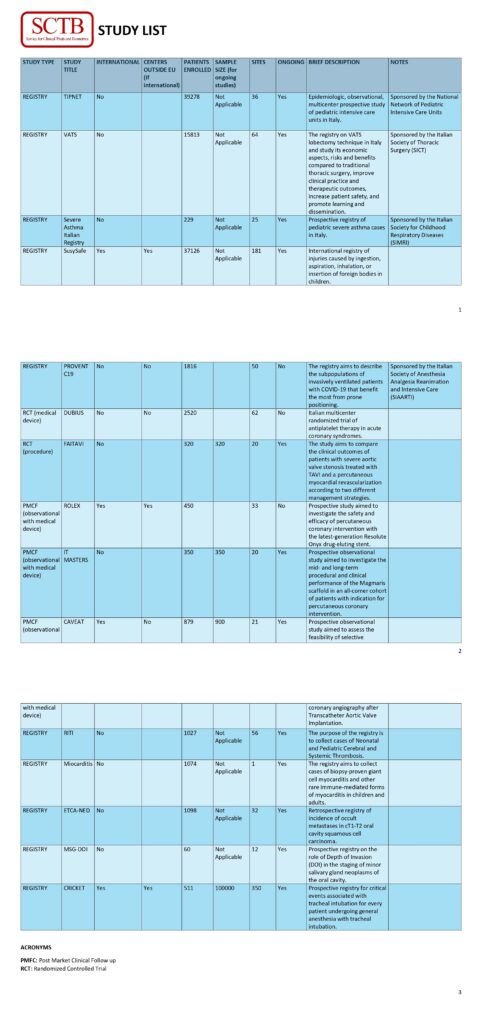
SCTB Studies: On the following page you will find all the studies that the service is carrying out
Other services:
UBEP has and offers advanced integrated IT services such as Microsoft Azure cloud services, including reserved storage space for its members (OneDrive) and the entire workgroup (SharePoint), personal, group and lab email, personal and shared calendar, automatic authentication to services, and connected computers (including Desktop, Laptop, and server). In addition to this, hosted on Unity’s Azure platform, virtual machines are enabled and activated for dedicated jobs and services, such as from MySQL data management servers, to the backend of Web sites and services, to high-performance computing for Deep Learning. In addition, UBEP also has five on-premise servers, 4 of which dedicated to internal users for RAM-optimized computation (up to 32 GB RAM per core, up to 16 cores per server). The other one (96 GB RAM x 16 cores) is devoted to R code development through an installation of the dedicated IDE RStudio Server Pro server and the computational backend of R-Shiny applications developed by the group and publicly available from the portal https://r-ubesp.dctv.unipd.it/.
Moreover, UBEP provides the possibility of creating (from scratch or models) personalized surveys or electronic case report forms (e-CRF) aimed at implementing electronic data capture (EDC) for clinical research, both for patient-reported outcomes and clinician-reported outcomes, with its deep expertise in managing REDCap.
REDCap (Research Electronic Data Capture) is an electronic data capture tool hosted at the Department of Cardiac-Thoracic-Vascular Sciences and Public Health. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources. The system is validated and it is compliant with FDA 21 CFR part 11. The data transmission is encrypted with a secure socket layer (SSL) technology and all changes to data are logged with a computerized timestamp in an audit trail.
The Unit of Biostatistics, Epidemiology, and Public Health provides access to the Department’s staff and students to use the web-based software “Covidence.” This software streamlines the production of systematic reviews by supporting citation screening, full-text review, risk of bias assessment, extraction of study outcomes, and the export of data and bibliographic references. Learn more at: https://support.covidence.org/help/unit-of-biostatistics-epidemiology-and-public-health-university-of-padova
Director: Prof. Dario Gregori
Via L. Loredan 18 – 35131 Padova – tel 049 8275646 e-mail: sctb.admin@ubep.unipd.it
